Main Process for The Medical Masks Production
•This article is from website, the copyright belongs to the original author or website.
With the development of the novel coronavirus epidemic situation, the shortage of medical protection supplies in the prevention and control in various areas of the world is still an urgent problem to solve, especially with the arrival of the counter-work tide, the pattern of epidemic prevention policies requires that production cannot be resumed without masks, and the shortage of masks is bound to for a longer period.
1. The basic structure and functions of disposable medical masks
Common medical masks are mainly composed of three layers of non-woven fabric. The inner layer is a common non-woven fabric, which is mainly used to absorb the moisture and moisture released by the wearer; the outer layer is a waterproof non-woven fabric, which is mainly used to isolate the liquid sprayed by the patient; the filter layer in the middle is used for the polypropylene melt-blown non-woven fabric treated with electret serves as a barrier against germs.

The core material of medical masks is polypropylene melt blown non-woven fabric after electret treatment *. It is important to remember these two keywords, as this is the key to filtering the new crown virus aerosol.
* The filtering mechanism of medical masks is Brownian diffusion, entrapment, inertial collision, gravity sedimentation and electrostatic adsorption. The first four are physical factors, that is, the characteristics of the non-woven fabric produced by the melt blown method, the filterability is about 35%; this is not up to the requirements of medical masks, we need to electrotype the material and let the fiber Charged and used static electricity to capture the aerosol where the new coronavirus is located.
2. Technical specifications and standard requirements for medical masks
Common testing standards for masks are as follows (China)
• EN 149 “Respiratory protective devices – Filtering half masks to protect against particles – Requirements testing marking”
• EN 14683 “Medical face masks – Requirements and test methods”
• ASTM F2100 “Standard Specification for Performance of Materials Used in Medical Face Masks”
• CFR 42 Part 84 “NIOSH Guide to the Selection and Use of Particulate Respirators”
• EN 14168 “Medical face masks. Requirements and test methods”
• GB 2626 “Respiratory protective equipment. Non-powered air-purifying particle respirator”
3. The formal production process of medical masks
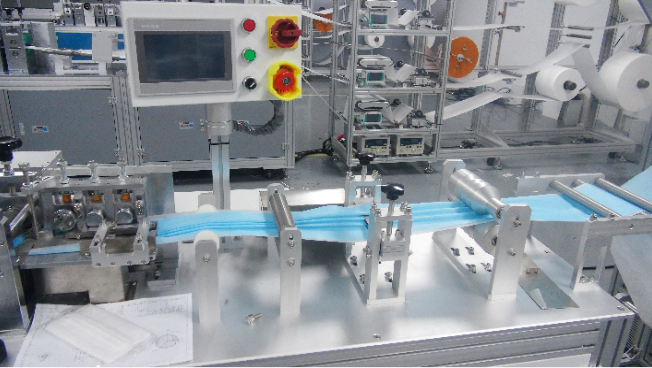
Hang the non-woven raw materials on the mask sheeter rack, and the machine will produce it automatically after debugging. The mask sheet will come out, and then the mask sheet will be transferred to the ear band machine for spot belting. After production, it is sterilized by ethylene oxide and left for 7 days to volatilize. The specific process is as follows:
1) Combine three layers of non-woven fabric
Three different non-woven fabrics were placed on the fixed support on the production line and neatly stacked together by the laminator above. There is a trumpet-shaped feeding port here, and a string of wires is continuously passed through the trumpet to the laminating machine.

2) Folding mask
Adult masks have uniform size specifications. How does it ensure that different people have the right size requirements to ensure that the nose, mouth and chin are wrapped to the greatest extent? This is due to the design shown below:

3) Stitch the metal wire fixed by the nose clip into the laminated three-layer non-woven fabric
From the suggestions for wearing masks correctly, we can know that the nose clip must be pinched to the bridge of the nose when wearing, so that wearing the mask will be firm. Otherwise, without this structure, my nose leak is more serious, the seal is not tight, and the protection effect is affected …
From the figure below, we can see that the wire is matched and conveyed along one side of the non-woven fabric, then the next edge is rolled, and the back is stitched, and the wire is stitched in.
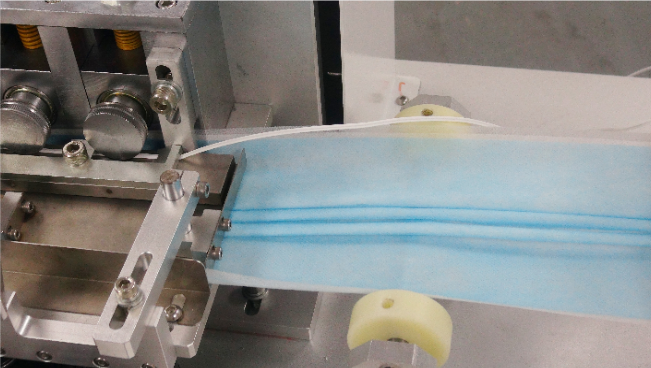
On the mask production line, there is a folding device to achieve this purpose.
Next, in order to create a crease and better processing in subsequent processes, it needs to be flattened by a roller machine.

4) Cut to a single mask unit
5) Fix the hanging ear-loop to the four corners of the mask,and weld the ear-loop
Because the process of wearing a mask, the hanging ear rope is in a relatively tight state for a long time. In order to strengthen the binding strength of the rope and the mask body, it is necessary to use adhesives at the four corners of the mask. During the pressing process, it is mechanically transported. Hang the ear rope, and fix the ear rope to the mask with an adhesive under the heat pressing device.
Through the above 6 steps, even if a flat disposable nonwoven mask is produced, it cannot be called a disposable medical nonwoven mask here, because there is still a very important link that has not been completed.

6) Disinfection
During the entire production process of the mask, not only the processing contact of the machine, but also the contact of many artificial links, the mask will inevitably be contaminated by bacteria. If it is an ordinary mask, it does not need to be sterilized, but the medical treatment requires the manufacturer to use a ring. Oxygen ethane (EO) sterilizer is disinfected. The mask was placed in an environment of 400 mg / L of ethylene oxide, and alkylation was applied to the hydroxyl group to make the microbial macromolecules inactive to achieve the purpose of sterilization. However, ethylene oxide is not only flammable and explosive, but also toxic to the human body. Therefore, it needs to stand for 7 days for analysis after disinfection. After the EO residual amount is lower than the required value, it can be packaged and shipped to medical staff.
Only medical masks produced according to the above process use high-melt melt-blown non-woven fabrics to produce filter materials, perform electret treatment to increase electrostatic adsorption, disinfect with ethylene oxide after production, and stand for 7 days to analyze ethylene oxide. Is a qualified, safe medical mask that can be used to isolate the transmission of new crown virus.
4. Equipment required for medical masks

For mask factories that produce disposable medical masks, the equipment needed includes mask forming machines, mask crimping machines, mask trimming machines, nose bridge line fitting machines, and earband spot welding machines.
This fully-automatic medical mask production line can work up to 100 pcs/min, per day under full-time operation. And it can be upgraded to an automatic line after the epidemic situation. 12,000 yuan can be changed back to the fully automatic line, which can reach a daily production capacity of 80,000.
Note: The production line equipment can only produce disposable flat masks, and cannot produce masks with three-dimensional or N95.
5. How to control the product quality and safety of disposable masks?
1) In view of the fact that the quality of filter materials for medical masks is difficult to control through convenient and effective inspection methods, the standardized operation of the production quality management system is the main means for enterprises to ensure the quality of mask products, so they are audited in the registration technology review and system assessment. Inspectors and inspectors will pay attention to the production process and source of supply of filter materials. Enterprises should control the filtering materials of products, clarify the sources and quality requirements of filtering materials, and have relatively stable production processes and sources of supply to ensure the quality of the products. Here, some reliable testing equipment is recommended for incoming inspection. Raw materials: Need to carry out sampling inspection on the raw non-woven fabric. The sampling process will use a circular sample cutterand fabric scale to determine the weight of the non-woven fabric, also measuring the waterproof(spraying rating tester) and breathable performance of the outer layer of the mask, and the moisture absorption of the inner layer. Breathability to determine whether the quality of the cloth can meet the requirements. And the packaging link: In determining whether the number of boxes is accurate, the factory generally checks by simple weighing.
2) The safety and effectiveness of medical masks are just some of the factors that play a protective role. The correct use and wearing method of the mask also directly affect the protection effect. Therefore, all the information required by the user should be clearly stated in the instructions to avoid misuse of the mask and reduce the risk of cross infection. For example, indicate the wearing method, clearly identify the front and back of the mask, suggest the use time, indicate the filter material level or related instructions, and so on. In addition, medical personnel have not yet clearly understood the scope of application of different types of medical masks. The scope of application of masks should be clearly stated in the instructions and the training of medical personnel should be strengthened.
3) Sterile medical masks are produced under the condition of a class 100,000 clean factory building, which is characterized by a maximum allowable number of dust particles equal to or greater than 0.5 microns and the number of particles must not exceed 3.5 million, and the number of particles greater than or equal to 5 microns cannot exceed 20,000. In addition, the maximum allowable number of microorganisms, the number of planktonic bacteria should not exceed 500 per cubic meter; the number of sedimentary bacteria should not exceed 10 per culture dish. At the same time, the pressure difference between clean rooms of the same cleanliness level is the same. For different cleanliness, the pressure difference between adjacent clean rooms is ≥5Pa, and between clean rooms and non-clean rooms is ≥10Pa. This is mainly to ensure that the air flows from the clean area to the non-clean area, and to avoid the backflow of airflow. The temperature is generally controlled at 20-22 ° C in winter; 24-26 ° C in summer; fluctuation ± 2 ° C. The humidity of clean room in winter is controlled at 30-50%, and the humidity of clean room in summer is controlled at 50-70%. When there are no special requirements for temperature and humidity, it is advisable to wear clean work clothes without producing a comfortable feeling. For its functional layout, equipment and management requirements, please refer to the “Guide to Inspection Points for Clean Room (District) of Medical Devices”.
6. Medical mask quality inspection
Primary medical masks can be sold in the market only after passing the test of specific items due to their characteristics of medical purposes. The testing of disposable medical mask products includes factory inspection and type inspection.
The factory inspection items should have at least the following items: appearance, structure and size, nose clips, mask bands, microbiological indicators, and ethylene oxide residues (if ethylene oxide sterilization is required).
The type inspection shall be the full performance inspection of the product standard.
